What is CAR-T therapy?
Chimeric antigen receptors (CAR) are synthetically engineered receptors that re-direct lymphocytes (usually T cells) to recognise and eliminate cells expressing a specific target antigen (1). T cells are an important component of the immune response (7). Therefore when the CAR binds to the target antigen the T cell becomes activated resulting in an anti-tumor response (1). Basically, CAR guides immune cells to target cancer cells causing a faster and more intense immune response.
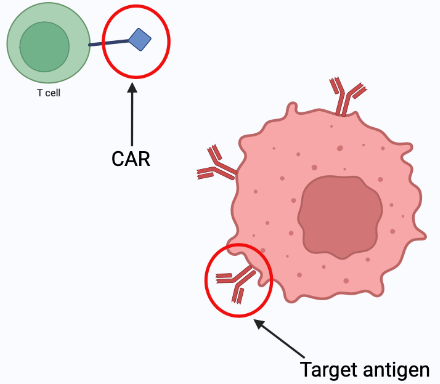
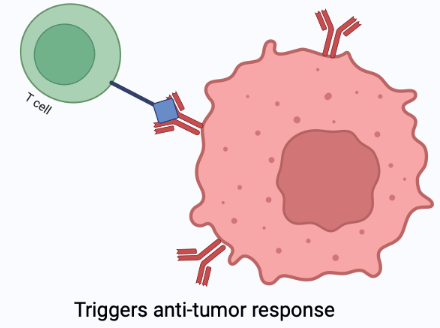
CAR-T therapy is not a totally new therapy. Since 2017 CAR-T therapy has been approved by the Food and Drug Administration (FDA) for use to treat blood cancers including lymphomas, some forms of leukemia and multiple myeloma (7). The current process of CAR-T therapy is customized to each patient and involves:
- Removal of blood from the patient to collect T cells (7).
- Re-engineering the T cells in the lab by inserting a gene into the T cells. The gene encodes the engineered CAR protein that is then expressed on the surface of the patient’s T cells, this creates the CAR-T cells (7).
- Millions of CAR-T cells are grown in the lab and are infused back into the patient (7).
- The CAR-T cells continue to multiply in the body and are directed to the cancer cells and kill them (7).
One of the core benefits of using CAR-T therapy instead of chemotherapy is the specificity of CAR-T therapy. Rather than killing all cells, it only targets the cancer cells (9).
‘CAR-T cells are now widely available in the United States and other countries and have become a standard treatment for patients with aggressive lymphomas’
Dr. Rosenberg (7)
CAR-T therapy and glioblastoma:
Glioblastoma is the most common form of brain cancer (8). It is a fast-growing tumor which is typically aggressive and challenging to treat: requiring surgery, radiotherapy and chemotherapy (2). Though CAR-T therapy has only been used clinically to treat blood cancers, two recent studies by Choi and colleagues and Bagley found administration of CAR-T resulted in the temporary shrinking of glioblastoma tumors (3,4). Though these changes were only temporary and they later returned, their results show promising results for the future (5).
CAR-T therapy and multiple sclerosis:
This year CAR-T therapy has just entered trials in the US to treat multiple sclerosis. Multiple sclerosis is an autoimmune disease that happens when immune cells (T and B cells) attack nerve cells. Currently, multiple sclerosis is treated by using antibodies that target CD20 (a protein on the surface of B cells), killing them but this process is slow and doesn’t always work (9). New research looks into how CAR-T cells which target CD19 (another protein on B cells) could be used to treat multiple sclerosis (9). CAR-T cells are much more efficient killers and are effective in penetrating tissue antibodies can’t get to (9).
The big picture:
Ultimately, the potential CAR-T therapy has in advancing cancer treatments, multiple sclerosis and other autoimmune diseases is huge. Though CAR-T therapy has been used to treat blood cancers its ability to target solid tumors is limiting and it can cause unpleasant and dangerous side effects. Despite this, exciting research reveals huge potential for the therapy… but we are at the start! More extensive trials will reveal the true potential of CAR-T therapies.
By Francesca Giannachi-Kaye
References:
- https://www.nature.com/articles/s41408-021-00459-7
- https://www.cancerresearchuk.org/about-cancer/brain-tumours/types/glioblastoma
- https://academic.oup.com/neuro-oncology/article/20/11/1429/4917531
- https://aacrjournals.org/clincancerres/article/25/7/2042/82575/Immunotherapy-for-Glioblastoma-Adoptive-T-cell
- https://www.nature.com/articles/d41586-024-00704-6#ref-CR1
- https://www.nature.com/articles/d41586-024-00470-5
- https://www.cancer.gov/about-cancer/treatment/research/car-t-cells
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10000604/
- https://www.nature.com/articles/d41586-024-00470-5